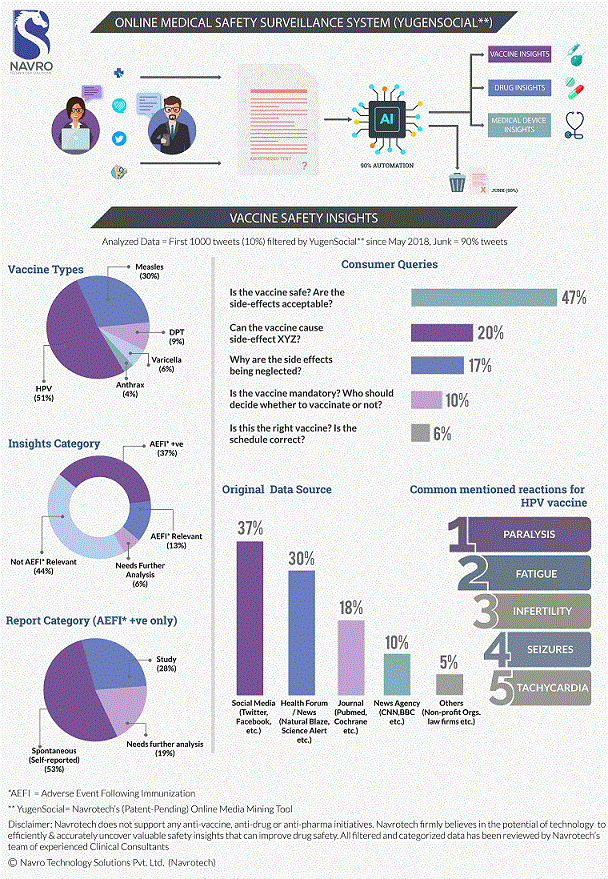
In the field of pharmacovigilance, vaccine safety surveillance plays a crucial role in monitoring and ensuring the safety of vaccines administered to the public. Vaccines are designed to prevent diseases and protect individuals from infections by stimulating the immune system. However, like any medication, vaccines can cause adverse reactions in some individuals.
Vaccine safety surveillance involves the continuous monitoring of vaccines after they have been licensed and introduced into the market. This process helps identify and assess any potential risks associated with vaccines, including rare or unexpected adverse events. By collecting and analyzing data on vaccine safety, regulatory authorities and healthcare providers can make informed decisions to ensure the continued safety and effectiveness of vaccination programs.
One of the key components of vaccine safety surveillance is post-marketing surveillance, which involves monitoring adverse events reported by healthcare professionals, patients, and vaccine manufacturers. Reporting systems such as the Vaccine Adverse Event Reporting System (VAERS) allow for the timely collection and analysis of data on vaccine-related adverse events. This information helps regulatory agencies like the Food and Drug Administration (FDA) and the World Health Organization (WHO) to detect potential safety issues and take appropriate actions if necessary.
In addition to passive surveillance systems like VAERS, active surveillance methods are also used to monitor vaccine safety. These methods involve conducting targeted studies or using electronic health records to assess the risk of adverse events following vaccination. By combining various surveillance techniques, healthcare authorities can obtain a comprehensive understanding of vaccine safety profiles and make evidence-based recommendations regarding immunization practices.
Overall, vaccine safety surveillance is essential for maintaining public trust in vaccination programs and ensuring that vaccines continue to be a cornerstone of disease prevention efforts worldwide. Through ongoing monitoring and evaluation, regulators can promptly address any emerging safety concerns and uphold high standards of patient care in immunization practices.
Enhancing Vaccine Safety: The Role of Pharmacovigilance in Early Detection, Trust Building, and Policy Support
- Early detection of rare or unexpected adverse events related to vaccines.
- Ensures continuous monitoring and evaluation of vaccine safety post-licensure.
- Helps maintain public trust in vaccination programs by addressing safety concerns promptly.
- Supports evidence-based decision-making for immunization policies and practices.
- Contributes to the overall effectiveness and success of vaccination campaigns in preventing diseases.
Challenges in Vaccine Safety Surveillance: Addressing Gaps and Biases in Pharmacovigilance
- Underreporting of adverse events may lead to incomplete data and hinder accurate risk assessment.
- Difficulty in establishing causal relationships between vaccines and reported adverse events due to confounding factors.
- Limited resources and capacity for comprehensive vaccine safety surveillance in some regions, leading to gaps in monitoring.
- Potential for bias in reporting adverse events, with healthcare professionals more likely to report severe cases or those that align with preconceived notions.
- Challenges in distinguishing between true vaccine-related adverse events and coincidental health issues, especially for commonly occurring conditions.
- Delayed detection of rare or long-term side effects that may not manifest until after extensive vaccine use.
- Lack of standardized criteria for defining and categorizing adverse events across different surveillance systems, leading to inconsistencies in data interpretation.
Early detection of rare or unexpected adverse events related to vaccines.
The proactive nature of vaccine safety surveillance in pharmacovigilance allows for the early detection of rare or unexpected adverse events related to vaccines. By continuously monitoring and analyzing data on vaccine safety, healthcare authorities can swiftly identify any unusual patterns or signals that may indicate a potential safety concern. This early detection enables prompt investigation, assessment, and appropriate actions to be taken to mitigate risks and ensure the continued safety and effectiveness of vaccination programs.
Ensures continuous monitoring and evaluation of vaccine safety post-licensure.
Ensuring continuous monitoring and evaluation of vaccine safety post-licensure is a critical pro of vaccine safety surveillance in pharmacovigilance. By actively tracking and assessing the safety profile of vaccines after they have been licensed and introduced to the market, healthcare authorities can promptly identify and address any potential risks or adverse events that may arise. This ongoing surveillance allows for timely interventions, if necessary, to maintain public confidence in vaccination programs and ensure the overall safety and effectiveness of vaccines in preventing diseases.
Helps maintain public trust in vaccination programs by addressing safety concerns promptly.
Vaccine safety surveillance in pharmacovigilance plays a crucial role in maintaining public trust in vaccination programs by addressing safety concerns promptly. By continuously monitoring and analyzing data on vaccine safety, regulatory authorities can quickly detect and investigate any potential adverse events associated with vaccines. This proactive approach not only ensures the safety and effectiveness of vaccination programs but also demonstrates transparency and accountability to the public. By addressing safety concerns promptly, vaccine safety surveillance helps build confidence in the healthcare system and encourages individuals to participate in immunization efforts, ultimately leading to better public health outcomes.
Supports evidence-based decision-making for immunization policies and practices.
Vaccine safety surveillance in pharmacovigilance plays a critical role in supporting evidence-based decision-making for immunization policies and practices. By continuously monitoring and analyzing data on vaccine safety, regulatory authorities and healthcare providers can make informed decisions regarding the introduction, use, and monitoring of vaccines. This proactive approach ensures that immunization policies are based on scientific evidence and real-world data, leading to more effective vaccination strategies and improved public health outcomes.
Contributes to the overall effectiveness and success of vaccination campaigns in preventing diseases.
Effective vaccine safety surveillance in pharmacovigilance significantly contributes to the success of vaccination campaigns in preventing diseases. By closely monitoring the safety profiles of vaccines, healthcare authorities can promptly detect and address any potential adverse events, ensuring that vaccines remain safe and effective for use. This proactive approach not only helps maintain public confidence in vaccination programs but also allows for the timely implementation of corrective measures to mitigate risks. Ultimately, by upholding high standards of vaccine safety through vigilant surveillance, healthcare providers can maximize the overall effectiveness of vaccination campaigns and safeguard public health against preventable diseases.
Underreporting of adverse events may lead to incomplete data and hinder accurate risk assessment.
One significant con of vaccine safety surveillance in pharmacovigilance is the issue of underreporting of adverse events, which can result in incomplete data and impede accurate risk assessment. When healthcare professionals, patients, or vaccine manufacturers fail to report adverse events following vaccination, important information regarding potential safety concerns may be missed. This lack of comprehensive data can create gaps in understanding the true safety profile of vaccines, making it challenging for regulatory authorities to assess risks accurately and make informed decisions. Addressing the underreporting issue is crucial to improving the effectiveness of vaccine safety surveillance systems and ensuring the timely identification and management of potential vaccine-related risks.
Difficulty in establishing causal relationships between vaccines and reported adverse events due to confounding factors.
One significant con of vaccine safety surveillance in pharmacovigilance is the challenge of establishing causal relationships between vaccines and reported adverse events due to confounding factors. Confounding factors such as underlying health conditions, concomitant medications, genetic predispositions, and environmental influences can complicate the process of attributing adverse events solely to vaccines. This complexity makes it difficult to definitively determine whether a reported adverse event is directly caused by a vaccine or if it is the result of other factors. As a result, healthcare authorities must carefully evaluate and analyze data from multiple sources to assess the true risk-benefit profile of vaccines and make informed decisions regarding their safety.
Limited resources and capacity for comprehensive vaccine safety surveillance in some regions, leading to gaps in monitoring.
One significant con of vaccine safety surveillance in pharmacovigilance is the limited resources and capacity in certain regions, resulting in gaps in monitoring. Inadequate funding, infrastructure, and trained personnel can hinder the ability to conduct comprehensive surveillance of vaccine safety. As a result, some regions may struggle to collect and analyze data effectively, leading to potential delays or underreporting of adverse events related to vaccines. These gaps in monitoring can compromise the timely detection of safety issues and the implementation of necessary interventions to protect public health.
Potential for bias in reporting adverse events, with healthcare professionals more likely to report severe cases or those that align with preconceived notions.
One significant con of vaccine safety surveillance in pharmacovigilance is the potential for bias in reporting adverse events. Healthcare professionals may be more inclined to report severe cases or those that align with preconceived notions, leading to an incomplete or skewed representation of vaccine safety data. This biased reporting can create challenges in accurately assessing the true risk-benefit profile of vaccines and may result in unnecessary concerns or misconceptions about their safety. It is essential for surveillance systems to address and mitigate reporting biases through rigorous data collection methods and thorough analysis to ensure a comprehensive understanding of vaccine safety profiles.
Challenges in distinguishing between true vaccine-related adverse events and coincidental health issues, especially for commonly occurring conditions.
One significant challenge in vaccine safety surveillance within pharmacovigilance is the difficulty in distinguishing between true vaccine-related adverse events and coincidental health issues, particularly for commonly occurring conditions. Due to the widespread use of vaccines and the prevalence of various health conditions in the general population, it can be challenging to attribute certain symptoms or illnesses directly to vaccination. This ambiguity can lead to confusion and uncertainty in identifying genuine vaccine-related adverse events, potentially causing delays in response and management of legitimate safety concerns.
Delayed detection of rare or long-term side effects that may not manifest until after extensive vaccine use.
One significant con of vaccine safety surveillance in pharmacovigilance is the delayed detection of rare or long-term side effects that may not manifest until after extensive vaccine use. Due to the limited duration of clinical trials before vaccine approval, certain adverse events with longer latency periods or low occurrence rates may go undetected until a large population has been vaccinated. This delay in identifying rare side effects can hinder timely intervention and management strategies, potentially leading to challenges in addressing unexpected safety concerns and maintaining public confidence in vaccination programs.
Lack of standardized criteria for defining and categorizing adverse events across different surveillance systems, leading to inconsistencies in data interpretation.
The lack of standardized criteria for defining and categorizing adverse events across different vaccine safety surveillance systems poses a significant challenge in pharmacovigilance. This inconsistency in data interpretation can result in varying reports of adverse events related to vaccines, making it difficult to compare and analyze data accurately. Without uniform guidelines for identifying and classifying adverse events, healthcare authorities may struggle to assess the true risk-benefit profile of vaccines and take appropriate measures to address safety concerns effectively. Standardizing criteria for defining adverse events is crucial to ensure the reliability and consistency of vaccine safety surveillance data, ultimately enhancing the quality of decision-making in immunization programs.